Hcooch Ch2 H2o: The Hydrolysis of Formate Esters
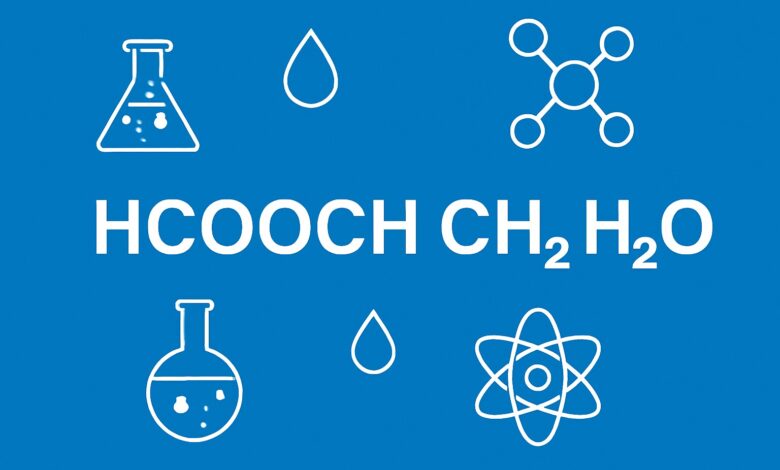
Chemistry is full of compounds that may look simple at first glance but hold deeper importance in both academic and industrial contexts. One such compound is HCOOCH CH2 H2O, which combines structural clarity with functional applications. Understanding the behavior, properties, and usage of this compound is essential for students, researchers, and industries working in the fields of organic chemistry and chemical engineering. This article explores HCOOCH CH2 H2O, covering its molecular composition, chemical behavior, applications, and significance.
Introduction to HCOOCH CH2 H2O
The compound HCOOCH CH2 H2O represents a chemical structure that integrates formate, methylene, and water groups. Each part of the formula contributes to its identity and role in reactions.
Breaking down the compound:
| Component | Chemical Role | Explanation |
|---|---|---|
| HCOOCH | Formate group | Acts as a functional base in organic chemistry. |
| CH2 | Methylene | Provides bonding versatility and stability. |
| H2O | Water molecule | Participates in hydration and reaction mechanisms. |
The presence of HCOOCH CH2 H2O in laboratory settings often allows chemists to explore reactions involving esters, hydration processes, and organic synthesis.
Structural Significance of HCOOCH CH2 H2O
The molecular arrangement of HCOOCH CH2 H2O is unique because of its ability to combine organic fragments with water molecules. This balance between hydrophilic and hydrophobic sections makes it interesting for reaction studies.
Key aspects of the structure:
-
Formyl group (HCOOCH): Provides reactivity for ester formation.
-
Methylene (CH2): Serves as a flexible chain extender.
-
Water (H2O): Functions as both solvent and participant in reactions.
This hybrid structure gives HCOOCH CH2 H2O importance in experimental organic chemistry.
Physical and Chemical Properties of HCOOCH CH2 H2O
The behavior of HCOOCH CH2 H2O can be better understood by analyzing its basic properties.
| Property | Description |
|---|---|
| Molecular nature | Organic compound with hydration characteristics. |
| Solubility | Dissolves in polar solvents like water and alcohol. |
| Reactivity | Involved in esterification, hydrolysis, and polymerization. |
| Stability | Stable under controlled temperature, reactive in acidic or basic media. |
These properties highlight why HCOOCH CH2 H2O is a compound of interest in both educational and industrial laboratories.
Role of HCOOCH CH2 H2O in Organic Chemistry
In organic chemistry, HCOOCH CH2 H2O plays multiple roles depending on the reaction setup. Its behavior often revolves around esterification and hydration, which are fundamental processes.
-
Esterification: The formate group in HCOOCH CH2 H2O enables ester bond formation.
-
Hydrolysis: The presence of water allows breakdown of complex molecules.
-
Polymerization support: Acts as a structural part of larger chemical chains.
Thus, chemists often use HCOOCH CH2 H2O as a model compound to study basic reaction principles.
Industrial Applications of HCOOCH CH2 H2O
Beyond academics, HCOOCH CH2 H2O finds its place in industries that depend on organic reactions and formulations.
| Industry | Application of HCOOCH CH2 H2O |
|---|---|
| Pharmaceuticals | Used in synthesis of intermediates and solvents. |
| Polymers & Plastics | Supports chemical bonding in polymer structures. |
| Agriculture | Potential role in formulation of fertilizers and pesticides. |
| Research | Used as a laboratory compound for organic studies. |
These applications demonstrate how HCOOCH CH2 H2O transitions from being just a chemical formula to becoming a tool for innovation.
Experimental Importance of HCOOCH CH2 H2O
Chemists working with HCOOCH CH2 H2O often use it to:
-
Study reaction pathways involving esterification and hydration.
-
Understand bond stability in mixed organic-inorganic compounds.
-
Explore solubility dynamics across different solvents.
Such experiments make it an excellent compound for advanced educational training.
Comparison of HCOOCH CH2 H2O with Similar Compounds
To understand its uniqueness, HCOOCH CH2 H2O can be compared with similar organic compounds.
| Compound | Similarity | Difference |
|---|---|---|
| HCOOCH3 (Methyl formate) | Shares ester properties | Lacks water interaction like HCOOCH CH2 H2O |
| CH3OH (Methanol) | Soluble in polar solvents | Does not exhibit ester characteristics |
| H2O (Water) | Hydration role | No organic ester group present |
This comparison shows that HCOOCH CH2 H2O has a distinct balance of ester reactivity and hydration, making it versatile.
Future Research on HCOOCH CH2 H2O
The growing demand for sustainable chemistry pushes researchers to explore compounds like HCOOCH CH2 H2O. Potential research directions include:
-
Designing eco-friendly solvents using this compound.
-
Developing biodegradable polymers with its structural role.
-
Studying green reaction mechanisms where water is naturally integrated.
Such pathways highlight how HCOOCH CH2 H2O can contribute to modern sustainable practices.
Educational Relevance of HCOOCH CH2 H2O
For students of chemistry, HCOOCH CH2 H2O serves as a practical example for:
-
Understanding functional groups in organic molecules.
-
Exploring reaction mechanisms that involve both organic and aqueous phases.
-
Learning how industrial applications stem from basic structural chemistry.
Thus, it connects textbook knowledge with real-world relevance.
Conclusion
The compound HCOOCH CH2 H2O may seem like just another chemical formula, but its significance in organic chemistry, industrial usage, and research makes it highly relevant. From esterification and hydration to its role in pharmaceuticals and polymers, it proves that even simple-looking compounds carry profound importance. As industries and laboratories continue to innovate, HCOOCH CH2 H2O will likely remain a key focus of study and application.
